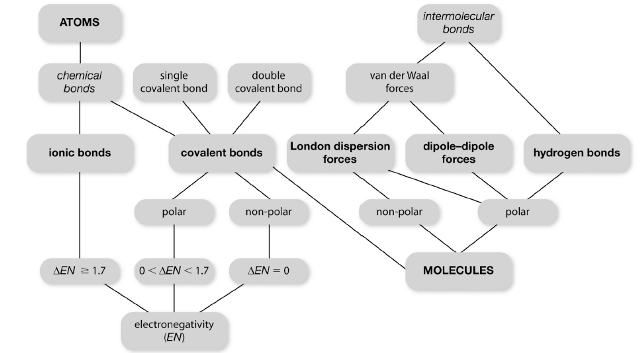
|
Study Guide for Unit 1.pdf Size : 131.916 Kb Type : pdf |

|
pdf answers to chapter 2 polymers.pdf Size : 565.339 Kb Type : pdf |

|
claire altered Carboxylic Acids and Esters.pdf Size : 169.608 Kb Type : pdf |

|
Organic Chemistry Reaction Mechanism for Thursday April 9 or Tuesday 14.docx Size : 73.115 Kb Type : docx |

|
Organic Chem reaction mech solutions.pdf Size : 220.796 Kb Type : pdf |

|
SCH4U polymerization mar 2015.docx Size : 60.211 Kb Type : docx |

|
1.4.1 and 1.5.1 Lab handouts.pdf Size : 228.743 Kb Type : pdf |

|
Activity3 Gaphing applications of alkanes and alcohol.pdf Size : 78.875 Kb Type : pdf |

|
Organic Summary Sheet.pdf Size : 8.271 Kb Type : pdf |

|
Ch 2 polymerization.pdf Size : 29.054 Kb Type : pdf |

|
Classifying Organic Compounds.pptx Size : 400.44 Kb Type : pptx |

|
Organic Chemistry Unit-2-2 (1) (3).pdf Size : 986.722 Kb Type : pdf |

|
ch 8 full solutions .pdf Size : 6008.073 Kb Type : pdf |

|
chem12_sm_08_5.pdf Size : 920.823 Kb Type : pdf |

|
chem12_sm_08_6.pdf Size : 854.03 Kb Type : pdf |

|
chem12_sm_08_8.pdf Size : 391.29 Kb Type : pdf |
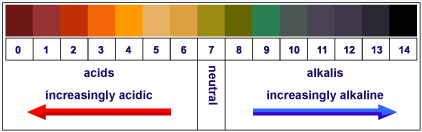

|
hess law questions f1.pdf Size : 2447.462 Kb Type : pdf |

|
chem12_sm_05_4.pdf Size : 265.945 Kb Type : pdf |

|
chem12_sm_06_5.pdf Size : 260.625 Kb Type : pdf |

|
enthalpy 5.2, 3 and 4 Student worksheet .docx Size : 84.209 Kb Type : docx |

|
chem12_sm_05_4.pdf Size : 265.945 Kb Type : pdf |

|
chem12_sm_05_3.pdf Size : 978.921 Kb Type : pdf |

|
chem12_sm_05_2.pdf Size : 2202.97 Kb Type : pdf |

|
Bare feet hot sand cool water.docx Size : 111.657 Kb Type : docx |

|
f1Introduction to Thermochemistry.pdf Size : 188.954 Kb Type : pdf |


|
apchapt9.ppt Size : 11281.5 Kb Type : ppt |

|
Molecular shapes_1551_VB.ppt Size : 13762.5 Kb Type : ppt |

|
pi bonds sigma bonds .pdf Size : 131.878 Kb Type : pdf |